Growth
Work Package L3
Essential driving forces employed for the growth process are concentration gradients across semipermeable interfaces, embodied in osmotic pressure and pH differences, lowering of interfacial tension, and a number of external stimuli aimed at achieving precise temporal and spatial control over the minimal growth system. Here, droplets are considered as the simplest model microcompartments and their growth will be realized at the expense of neighboring droplets. In the case of lipid vesicles, growth will rely on fusion as a means of supplying additional membrane material. Polymersomes on the other hand will increase their size after the integration of membrane constituents, released on demand, which mimics natural cell growth and, to our knowledge, has not been demonstrated so far.
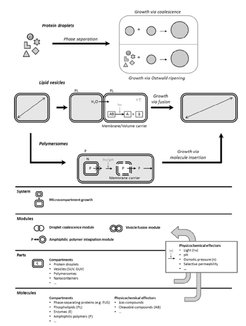
Due to the fact that self-replication is a fundamental process, characteristic for living entities, the Work Package L3 will focus on the realization of growing microcompartments as a prerequisite for the establishment of self-replicating systems. Unlike the more conventional interpretation of the term “growth”, which encompasses the entire process of proliferation and is population-related, growth in this context refers solely to the increase of an individual microcompartment volume (and interface) and is considered as a process within a synthetic cell cycle. The newly designed Work Package L3 will largely benefit from expertise, gained during the first funding phase, but it will engage the existing methods and knowledge in a different context. Unlike natural cellular growth, which involves very complex biochemical interactions and machineries, we aim to establish a minimal system, featuring growth, based on much simpler physicochemical mechanisms. Essentialdriving forces for the growth process will be concentration gradients across semipermeable interfaces, embodied in osmotic pressure and pH differences. In addition, light and other stimuli will be used as an external, immediately applicable driving force to achieve precise temporal and (potentially) spatial control over the minimal growth system. Analogous to the general logic, droplets are considered as the simplest model microcompartments and their growth will be realized at the expense of neighboring droplets. In the case of vesicles, which are more realistic approximation of living cells, growth will rely on fusion as a means of supplying additional membrane material. Thus, phenomena like droplet coalescence or vesicle fusion will be employed in a controlled manner for a conceptually novel purpose. Polymersomes on the other side will increase their size after the integration of membrane constituents, released on demand, which mimics natural cell growth and, to our knowledge, has not been demonstrated so far. The long-term goal will be to combine the growing containers as a functional module with cell polarization and/or division modules (to be developed in Work Package L4) and also integrate them into a morphogenic membrane system (to be developed in Work Package L5).
