Research
Microfluidics, compartments assembly, energy modules, division, signalling and motility of synthetic cells
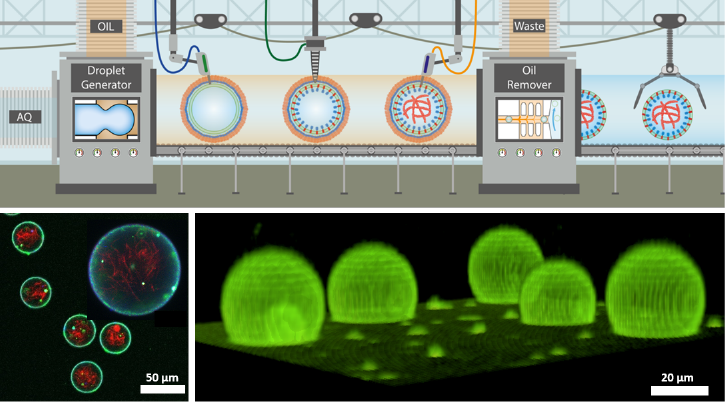
With the ultimate aim to construct a life-like eukaryotic cell, we follow a bottom-up synthetic biology approach for reconstituting cellular phenomena in vitro – disentangled from the complex environment of a normal cell. To address this, we developed a practical and highly tuneable method for the high-throughput on-demand creation of synthetic cell model systems in the form of droplet-stabilized giant unilamellar vesicles (dsGUVs), multicompartment systems and free-standing GUVs (Work Package Compartments). To achieve this, we used droplet-based microfluidics to encapsulate SUVs in polymer-stabilized water-in-oil droplets. By tuning the charge of the inner droplet interface, the adsorption of lipids can either be inhibited, leading to multicompartment systems or induced, leading to the formation of a droplet-supported GUV or a combination of the two. Moreover, we developed and optimized a break-through, highly controllable picoinjection microfluidic technology for the bottom-up assembly of functional compartments. This technology allowed us successful incorporation of the functional energy and adhesion-associated protein complexes (e.g., F0F1-ATPases, proteorhodopsinαIIbβ3integrin-, talin head, G actin, α-actinin, tubulin, kinesin and myosin) into dsGUV and free-standing GUVs (Work Packages Metabolism, Signaling and Motility).
For the signalling and motility activities (Work Package Signaling and Motility), picoinjection technology was used to enable the sequential and precise delivery of controllable amounts of adhesion and motility protein complexes into micrometer-sized compartments. It is worth noting that these compartments and protein complexes do not assemble correctly by simply mixing the components – in other words, we have managed to combine, simultaneously and under identical conditions, several biomolecular systems that do not function together naturally by purely mixing them! Therefore, a real bottom-up, step-by-step construction of a complex synthetic cell with desired features has been accomplished. To validate the functionality of the reconstituted adhesion-associated protein complexes the adhesion/spreading behaviour of the functionalized GUV- and liposome-based synthetic cell systems on surfaces coated with native or synthetic extracellular matrices was investigated. In addition to adhesion we showed that the developed synthetic cells are capable of self-assemble different cytoskeletal and transmembrane proteins, and, as a consequence, generate cellular functions such as migration and self-propelling. Moreover, for the signalling and motility activity we designed and implemented a new concept of synthetic proteins which is based on bispecific ankyrin repeat proteins, so-called DARPins. Implementation of such DARPins in synthetic cells is of enormous interest as they may enable us to further reduce the biological complexity by restricting the protein function to its binding capability.