
Research of the Robinson Group
Mimicking eukaryotic compartments with synthetic systems aided by microfluidic tools
Multi-compartment lipid membrane systems

Eukaryotic cells have evolved membrane-bound sub-cellular organelles. The advantage of which is to provide spatial organisation of biomolecules, therefore allowing more diverse cellular functionalities., The Robinson lab uses giant unilamellar vesicles (GUVs) as tools to not only mimic the outer cell membrane, but we also encapsulate smaller GUVs to mimic membrane-bound organelles. These multi-compartment vesicles also called ‘vesosomes’ can be tailored to contain specific biomolecules within each compartment or to contain functionalised lipids for specific applications. By creating these artificial eukaryotic cell-like systems, we hope to gain valuable insights into how biological cells function - namely self-organisation and multiple enzymatic reactions
Microfluidic systems to create giant unilamellar vesicles

Production via traditional bulk method is unreliable, efficient and lacks control. We therefore use microfluidic technology to create our GUVs via water-in-oil droplets. Double emulsion templates are produced using microfluidic droplets at high-throughput rates (kHz), and the organic phase is later removed leaving highly mono-disperse microfluidic GUVs (or mGUVs). Compared to bulk methods, the encapsulation efficiencies are high and the sizes of the mGUVs can be precisely tuned from 10 to 150 microns. One of our goals is to produce pure-lipid surfactant-free vesicles to more accurately mimic real biomembranes.
Microfluidic platforms for precise handling and analysis of synthetic cells
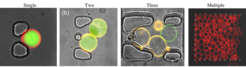
While GUVs are powerful tools for creating biomimetic synthetic cells, the handling and subsequent imaging of them is not always straightforward. Therefore, we make use of microfluidic platforms which are designed to capture the vesicles in specific spatial locations for on-chip analysis. Depending on the application, we can immobilise defined numbers of GUVs (from single vesicles to 100s) and due to the microfluidic flow control, we are still be able to add or remove molecules surrounding them. This is particularly advantageous for drug delivery or biophysical applications, but is also essential for triggering events externally in the context of mimicking cell processes. Having recently published a high-capacity device for high-throughput vesicle analysis, current efforts within the group are focussed on the next generation of devices for multiple high-throughput experiments and on a single chip.


